More Michigan residents file lawsuits in meningitis outbreak
At least five more people sickened in Michigan in the recent outbreak of fungal meningitis have filed lawsuits, the Detroit Free Press reported.
The new lawsuits follow a class-action lawsuit filed in Detroit federal court last week on behalf of any Michigan resident who may have been exposed to tainted steroids from the New England Compounding Pharmacy in Massachusetts. The pharmacy manufactured the steroids that became contaminated with fungus.
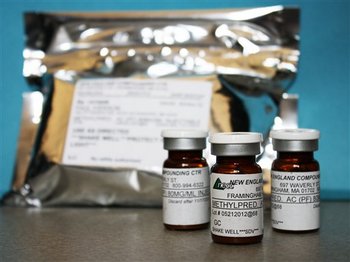
In this photo made available, Oct. 9, 2012, the Minnesota Department of Health shows shows vials of the injectable steroid product made by New England Compounding Center implicated in a fungal meningitis outbreak that were being shipped to the CDC from Minneapolis.
AP photo
The Centers for Disease Control has linked 308 illness and 23 deaths across the country to the contaminated steroids, according to The Associated Press.
In Michigan, the toll is six deaths and 69 infections. The Michigan Department of Community health says the deaths include a 62-year-old man and a 78-year-old woman in Washtenaw County, a 79-year-old Oakland County woman, a 56-year-old Genesee County woman, and a 67-year-old Livingston County woman, AP reported.
Indiana authorities also report the death of an 89-year-old woman from Cassopolis, Mich., who was treated at an Indiana clinic.


Comments
PineyWoodsGuy
Thu, Oct 25, 2012 : 4:50 a.m.
Just Wondering. Do Big Name Pharma companies "license" independent pharma compounders to manufacture their drugs? If so, then Patient-Rights "Transparency" Commands they be disclosed! Are the "licensees" Overseas? Can the patient "Check the Name of the Manufacturer on the Bottle" before the patient consents to injection??? Inquiring Minds Should Like to Know. Dig? (Are you Injecting Doctors Listening???).
justcurious
Wed, Oct 24, 2012 : 2:51 p.m.
The company was shipping out the medication before receiving the sterility results on the batches. It makes you wonder how often this happens. Maybe the hospitals and clinics using the medicine should have access to those results before giving the medication to patients.
shipdog7
Wed, Oct 24, 2012 : 1:59 p.m.
i have had those shots a couple of times now. The last Feb 22nd 2012. Not at one of the sites mentioned to have the tainted medicine. Everyone reacts differently to them as far as pain goes. Some last for a year or more. Some a few months or less. Just prior to the news breaking on this problem I was considering making an appointment for another round of shots. Even though I get them at a well known hospital who apparently doesn't use the Compounding Center., needless to say this whole thing makes me more than anxious.