Investors concerned FDA changes could undermine medical device investments
Michigan venture capitalists are keeping a watchful eye on regulatory changes at the U.S. Food and Drug Administration even as information technology is injecting innovation into the medical device industry.
Experts gathered last week at the Michigan Growth Capital Symposium said uncertainty about pending changes at the FDA could alter investment strategies.
“You have products with increasing complexity going through a regulatory system where the pendulum has swung way over to the safety side,” said John Neis, managing director of Venture Investors, which has an Ann Arbor office. “It’s much more difficult to get products through.”
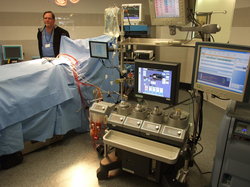
Medical devices, like this heart-lung machine produced in Scio Township by Terumu Cardiovascular Systems, could face additional regulations by the Food and Drug Administration.
Nathan Bomey | AnnArbor.com
The FDA is said to be considering placing additional regulatory requirements on medical device companies, an industry that is creating jobs for the Ann Arbor region.
Device firms like HandyLab, which was acquired last fall for $275 million by Becton, Dickinson and Co., and Accuri Cytometers, which topped $10 million in revenue in 2009, are among the companies that could be impacted by new regulations.
“The FDA is a big risk for all of us in health care,” said Jan Garfinkle, managing partner of Ann Arbor-based VC firm Arboretum Ventures, which invested in HandyLab and Accuri. “We’re not sure what it’s going to mean.”
Complicating the equation is uncertainty about how federal regulators will approach new health care technologies like remote monitoring and diagnostics - areas that are quite attractive to investors.
“We’re now inventing technologies and approaches to health care and health maintenance where there (is no precedent),” said Laura Heisler, director of programming for the Wisconsin Alumni Research Foundation. “The FDA just hasn’t seen (those technologies) before. Guidance documents, for example, don’t exist for a lot of these things.”
Several investors said they prefer to fund life sciences companies whose medical devices are classified under the 510(k) category. Under that group, companies must notify the FDA in advance of their device hitting the market, but they don’t have to get preapproval before selling the device.
Changes to FDA regulations could alter investment strategies, but that doesn’t intimidate Immanuel Thangaraj of global VC firm Essex Woodlands Health Ventures.
“We will eventually see clarity with what’s going on at the FDA,” he said. “It will no doubt be more challenging but when we figure out what that path is, we’ll figure out a way to bring back innovation.”
Contact AnnArbor.com's Nathan Bomey at (734) 623-2587 or nathanbomey@annarbor.com. You can also follow him on Twitter or subscribe to AnnArbor.com's newsletters.


Comments
JD
Sun, May 16, 2010 : 3:27 p.m.
...have a product with problems or cannot be approved. We are all in this together... to make safe, effective, affordable products that can both help people and provide a business profit. All are equally key elements for business success. We need a better system to generate good ideas, evaluate the potential of each, filter and cut lose the losers sooner (most), and rapidly provide the evidence necessary for optimizing advancement to the marketplace. Doing this would make it easier for the FDA to review. We cannot blame the FDA for industry shortcomings. I will also add that the users (ie, physicians, nurses, etc.) are partially to blame for this call for increased FDA scrutiny. Sometimes they need to take more time to understand the products they are using. I can't tell you how many times you go to an inservicing and don't get the attention that is needed. Many product complaints are a result of user error, not because of a faulty device. (Yes, I understand their side, and the limited time they have to meet the demands of the day.)
JD
Sun, May 16, 2010 : 3:03 p.m.
The present scrutiny of the FDA by special interest lobby groups (ie, tort lawyers) represent one side of the review process issue, whereas the other side is represented by patient advocacy or business lobby groups. Some internal mismanagement, inconsistencies and errors by the FDA hasn't helped, but overall the FDA reviewers have done a very good job in the 20+ years I have worked in this industry. Despite my chagrin of having my expertise and authority questioned, it is the FDA's charter to protect the public that drives approval, and I must say that their questions have had much merit. In fact, I would be surprised if most persons in the RA field didn't often ask themselves why more insightful FDA questioning was not made. I can list off quite a few products that the FDA has given lenient discretionary approval. I would suspect most VC executives just want to get by the regulatory process, regardless of the risk to patients, knowing that they will soon sell off their future business risks for a good profit. VC people make a lot of money when most of the real credit should go to the people in the trenches that have made medical technology possible. The VC belly aching I suspect is just a strategy move, and someone will always be there when there is the smell of money. I see the main problem is that it costs too much money, time and resources to only find in the end that you